Nobel Prize in Chemistry 2017 has been awarded on Wednesday October 4, 2017 by the Royal Swedish Academy of Sciences to three biophysicists Jacques Dubochet, Joachim Frank and Richard Henderson, “for developing cryo-electron microscopy for the high-resolution structure determination of biomolecules in solution“. Cryo-electron microscopy is “a cool method for imaging the materials of life”, or for imaging molecules of life. Cryo-electron microscopy or Cool microscope technology revolutionises biochemistry, it simplifies and improves the imaging of biomolecules. The development allows scientists to visualize proteins and other biological molecules at the atomic level.
Jacques Dubochet, 75 born in Aigle, Switzerland a Swiss citizen, is a professor at the University of Lausanne in Switzerland. Joachim Frank, 77 born in Germany and now a U.S. citizen, is a Columbia University professor in New York. Richard Henderson, 72 born in Edinburgh, Scotland, Cambridge University, UK is Programme Leader, MRC Laboratory of Molecular Biology, Cambridge, UK.
A picture is a key to understanding. Scientific breakthroughs often build upon the successful visualisation of objects invisible to the human eye. However, biochemical maps have long been filled with blank spaces because the available technology has had difficulty generating images of much of life’s molecular machinery. Cryo-electron microscopy changes all of this. Researchers can now freeze biomolecules mid-movement and visualise processes they have never previously seen, which is decisive for both the basic understanding of life’s chemistry and for the development of pharmaceuticals.
Electron microscopes were long believed to be only suitable for imaging dead matter, because the powerful electron beam destroys biological material. But in 1990, Richard Henderson succeeded in using an electron microscope to generate a three-dimensional image of a protein at atomic resolution. This breakthrough proved the technology’s potential.
Joachim Frank made the technology generally applicable. Between 1975 and 1986 he developed an image processing method in which electron microscopes’ fuzzy two dimensional images are analysed and merged to reveal a sharp three-dimensional structure.
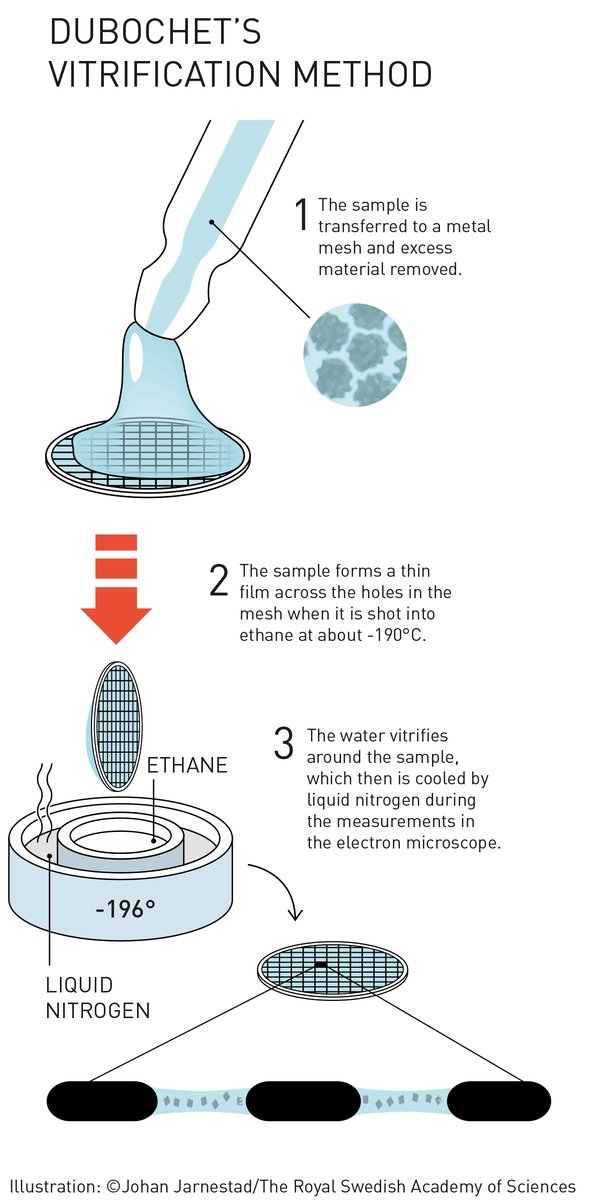
Jacques Dubochet added water to electron microscopy. Liquid water evaporates in the electron microscope’s vacuum, which makes the biomolecules collapse. In the early 1980s, Dubochet succeeded in vitrifying water – he cooled water so rapidly that it solidified in its liquid form around a biological sample, allowing the biomolecules to retain their natural shape even in a vacuum.
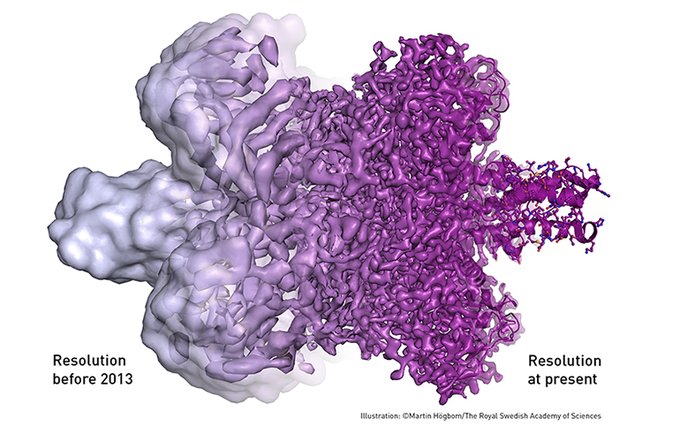
Following these discoveries, every nut and bolt of the electron microscope has been optimised. The desired atomic resolution was reached in 2013, and researchers can now routinely produce three-dimensional structures of biomolecules. In the past few years, scientific literature has been filled with images of everything from proteins that cause antibiotic resistance, to the surface of the Zika virus. Biochemistry is now facing an explosive development and is all set for an exciting future. U. S. Scientists used cryo-electron imaging to quickly determine the shape of Zika virus and have succeeded in getting details of the Zika virus’s surface, once it was identified as the cause of severe birth defects. The findings could help scientists eventually speed up research into vaccines and develop a vaccine for the virus. In January, Rossmann and his colleagues had announced that they had located the envelope proteins that enable Zika to meld with the host cells it infects, and according to Rossmann, the microscopy field is entering a period of “resolution revolution.” That is, scientists are scanning molecules in finer and finer detail.
To see the structure of molecules at ultrahigh resolution, scientists must hold molecules in place in their natural configuration. Other microscopic techniques, such as X-ray crystallography, are far more rigid than cryo-electron microscopy. The technique flash-freezes a sample to create a layer of ice like a pane of glass over a layer of liquid where the molecules can retain their natural shape.
Dubochet figured out how to cool water so quickly that crystals would not form in the early 1980s and first submitted for publication, “Discovery of water vitrification and development of cryo-electron microscopy”, it was rejected — the publishers did not believe water could be manipulated this way.
According to Frank, “Cryo-electron microscopy is about to completely transform structural biology,” as he created three-dimensional pictures from electron microscopes’ two-dimensional images. For him, he said, the “coolest molecule has always been the ribosome.” The ribosome, a cluster of RNA and protein, is tiny and hard to image. Its width is less than the wavelength of visible light. Cryo-electron microscopy allowed Frank and his colleagues to view the camera-shy particle.
Henderson had in 1990s shown that cryo-electron microscopy could be as detailed as X-ray crystallography when he made an atomic model of a member protein found in microorganisms.